Retatrutide
Retatrutide is an investigational once-weekly injectable being developed by Eli Lilly and Company. Unlike many approved medications, as of now Retatrutide has not been approved by Health Canada for commercial sale.
Its potential indications (under study) are:
- Obesity and overweight management – the primary indication under development. In Phase 2 trials, retatrutide produced robust weight reduction in adults with obesity or excess body weight. Multiple Phase 3 studies (the TRIUMPH program) are now ongoing to confirm efficacy, safety, and long-term outcomes.
- Type 2 diabetes with obesity – being studied in Phase 3 as part of broader obesity trials to assess improvements in glycaemic control and metabolic health in people who also have type 2 diabetes.
- Metabolic dysfunction–associated steatotic liver disease (MASLD / NAFLD) – promising Phase 2 data showed over 80% mean reduction in liver fat with higher doses, but this indication has not yet entered Phase 3. Future studies may explore this potential use.
The ongoing Phase III program, which began on 28 August 2023 and is expected to continue until 6 February 2026, will determine whether these potential indications translate into formal regulatory approvals.
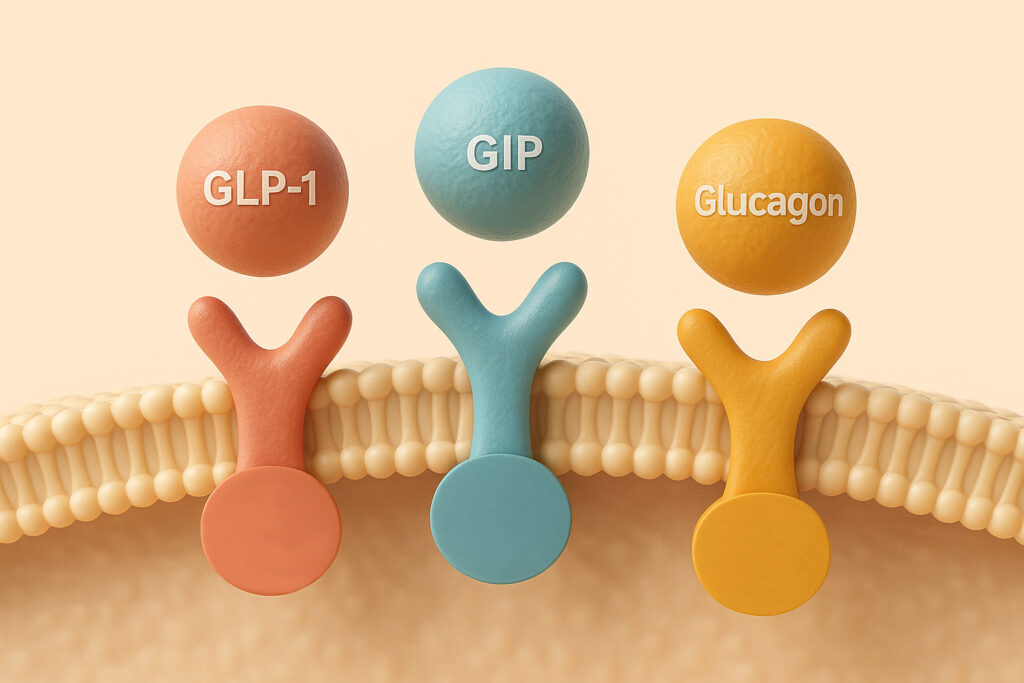
Mechanism of action: Retatrutide is a triple-agonist targeting GLP-1 (glucagon-like peptide-1), GIP (glucose-dependent insulinotropic polypeptide) and glucagon receptors. By acting on these three pathways, it suppresses appetite, increases fullness, slows gastric emptying and may increase basal energy expenditure (via glucagon receptor activation) – making it one of the more ambitious candidates in weight-management pharmacotherapy.
Buying Retatrutide Online
Because Retatrutide has not yet been approved for general use in Canada, legitimate telemedicine platforms that might list it are not offering “approved prescriptions” for it at the moment. Buying Retatrutide online through normal channels in Canada is not yet possible legally – only through enrolment in clinical trials. According to reports, some websites claim to offer “pre-orders” or “compounded retatrutide” pens for sale to Canadian residents, which raises serious regulatory and safety flags. If and when Retatrutide becomes approved, one would expect a telemedicine model similar to other weight-management injectables: an online questionnaire, virtual consultation, prescription issuance and home delivery. But for now, “buying online” is limited to clinical-trial frameworks, not open retail.
Retatrutide Without a Prescription?
In Canada, Retatrutide is not yet approved by Health Canada and therefore has no defined prescription status. When (and if) Health Canada grants marketing authorisation, it will almost certainly be prescription-only, in line with regulatory precedent for injectable incretin-based therapies used for obesity and type 2 diabetes. Until approval, any retail offer or “no-prescription” sale to Canadians is outside the legal supply chain and should be treated as unsafe.
Prices in Canada
As of now, Retatrutide has not been approved by Health Canada, and therefore no official retail price is available in Canadian pharmacies. Pricing information will only be released once the medication receives regulatory approval and enters the market.
Typically, newly approved injectable medications for chronic weight management in Canada are prescription-only and become available through pharmacies after Health Canada authorization, followed by pricing review by the Patented Medicine Prices Review Board (PMPRB) and assessment for public or private insurance coverage.
Until then, any pricing listed online should be considered unverified and not associated with official Canadian distribution channels.
Use and Outcomes
In Phase 2 clinical studies, retatrutide – a once-weekly injectable triple agonist acting on GIP, GLP-1, and glucagon receptors – demonstrated substantial and multi-system benefits in adults with obesity, with or without type 2 diabetes.
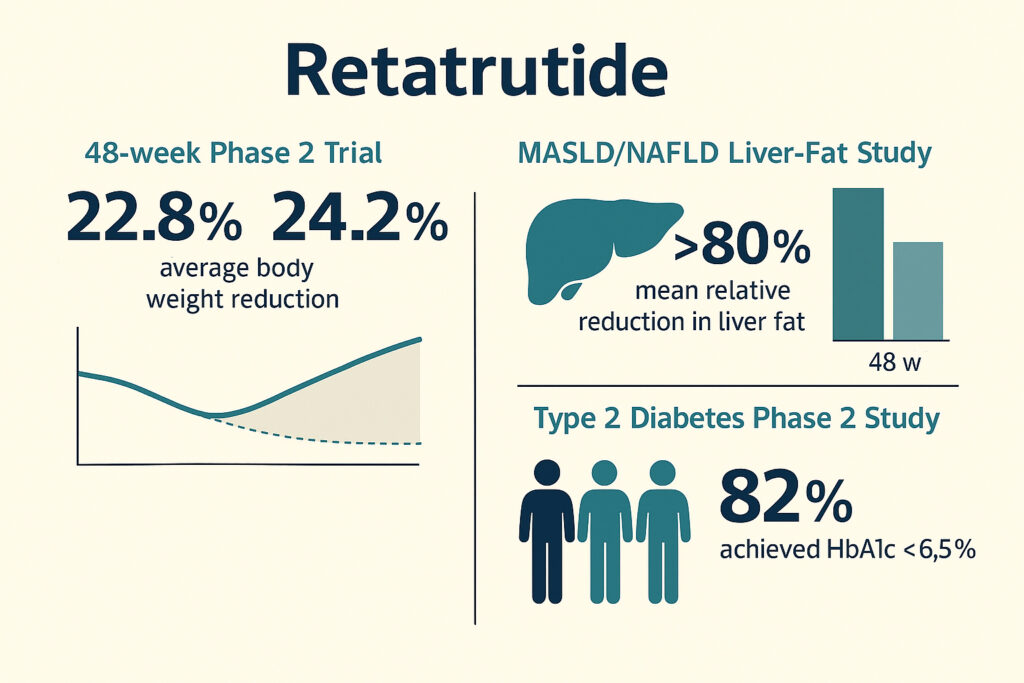
In the main 48-week Phase 2 trial (NEJM, 2023), participants receiving 8 mg and 12 mg weekly achieved mean body-weight reductions of 22.8% and 24.2%, respectively. By week 36, weight loss had already reached about 17%.
In a dedicated liver-fat substudy among participants with metabolic dysfunction–associated steatotic liver disease (MASLD/NAFLD), retatrutide produced marked reductions in hepatic fat: the mean relative reduction exceeded 80% with 8 mg and 12 mg doses at 24 and 48 weeks, and over 85% of participants achieved normalization of liver fat (<5% by MRI-PDFF).
In another Phase 2 study involving adults with type 2 diabetes, retatrutide (4 mg–12 mg) significantly reduced HbA1c compared with placebo and dulaglutide 1.5 mg; up to 82% of participants achieved HbA1c < 6.5%, and about 31% reached HbA1c < 5.7%.
If approved, retatrutide would be administered by weekly subcutaneous injection with dose escalation under medical supervision. Its mechanism – combining GLP-1, GIP, and glucagon receptor activity – suggests broader metabolic effects than current GLP-1-only agents. However, long-term safety and durability of these results remain under evaluation in ongoing Phase 3 trials.[1]
Side Effects and Contraindications
Data from the Phase II trial of Retatrutide show that the most frequent side effects were gastrointestinal. Participants often reported nausea, vomiting, diarrhoea, or constipation, particularly during the first weeks of treatment and at higher doses. These effects were usually mild to moderate and tended to decrease over time. Some people also mentioned headache, dizziness, and fatigue, which are common for drugs acting on GLP-1 pathways. Injection-site reactions were minor and transient. As with other incretin-based injectables, there were isolated cases of pancreatitis noted during trials, though no consistent pattern of severe liver or kidney toxicity was observed. Hypoglycaemia appears uncommon unless Retatrutide is combined with insulin or sulfonylureas.
Because Retatrutide is still investigational, formal contraindications have not yet been issued by Health Canada. However, class warnings from related GLP-1 and GIP agonists are expected to apply. These include:
- History or risk of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia type 2 (MEN2);
- Previous pancreatitis or severe gastrointestinal disease such as gastroparesis;
- Pregnancy and breastfeeding, due to lack of data;
- Avoidance of concurrent use with other GLP-1 receptor agonists.
Overall, Retatrutide’s emerging safety profile resembles that of existing incretin therapies – manageable for most users but requiring careful medical supervision once approved.
Experience of People
Participants in Retatrutide clinical studies have reported promising outcomes in controlled research settings, while real-world access remains unavailable pending regulatory approval.
Eli Lilly’s Phase 2 trial showed participants on the highest dose achieved a mean weight reduction of 24.2% over 48 weeks.
One participant from the ongoing TRIUMPH-1 Phase 3 trial shared their personal experience on Reddit:
- “After 80 weeks I am finished with my Retatrutide clinical trial. I was in the TRIUMPH 1 trial which was for pure obesity… My starting BMI was 34.7 and it’s now 23.2!!! I’ve lost 74 lbs — from 240 lbs to 166 lbs!”[2]
For now, real-world experience remains limited and conditional, and any use of Retatrutide should occur only within authorised clinical trials or following formal Health Canada approval.
Alternatives
While Retatrutide remains under investigation, several prescription medications are already approved in Canada for long-term weight management. These options differ in their mechanisms, cost, and availability, but all require medical supervision.
GLP-1 receptor agonists are currently the most widely used class
- Semaglutide (Wegovy) and liraglutide (Saxenda) help regulate appetite and improve blood sugar control. These drugs are injected once weekly (semaglutide) or daily (liraglutide).
- Another option is Orlistat (Xenical), an oral medication that reduces fat absorption in the gut. Although less potent, can be used by individuals who cannot take injectable therapy.
- Tirzepatide (Mounjaro, Zepbound), a dual GIP/GLP-1 agonist, was recently approved in Canada for obesity and type 2 diabetes. Its results are among the strongest currently seen, with mean weight loss reaching up to 20% in trials.
Each of these treatments has specific safety considerations and is not suitable for everyone. Medical assessment is essential to determine which therapy best aligns with individual health status, lifestyle, and treatment goals. Once Retatrutide becomes available, it is expected to join this group as a next-generation multi-agonist therapy offering broader metabolic effects.
FAQ
No. As of now, Retatrutide has not been approved by Health Canada. It remains an investigational drug in Phase III clinical trials.
No legal source exists yet in Canada. Any site claiming to sell or “pre-order” Retatrutide is not authorised and may offer unsafe or counterfeit products.
Almost certainly yes. All injectable incretin-based medications for obesity and diabetes in Canada are prescription-only. Retatrutide will almost surely follow that path.
No. Combining Retatrutide with semaglutide, liraglutide, tirzepatide or similar incretin drugs is not recommended, due to overlapping mechanisms and risk of side effects.
Wegovy acts only on GLP-1, Mounjaro targets GIP + GLP-1, while Retatrutide activates GLP-1 + GIP + glucagon – a broader metabolic reach that may explain stronger weight-loss signals, though long-term data are still pending.
References
-
1. 85th Scientific Sessions. Phase 2 trial results demonstrate benefits of retatrutide in obesity, type 2 diabetes, NASH [July 11, 2023]. Retrieved November 7, 2025
-
2. Reddit. Retatrutide clinical trial done! Now onto Zep! Retrieved November 8, 2025